Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species possesses a formal charge?
A) BH3
B) BH4
C) CCl4
D) H2S
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following bonds is the most polar?
A) F-F
B) H-F
C) C-H
D) C-Si
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are there in the valence shell of the carbon atom of the methyl anion, CH3-?
A) 2
B) 4
C) 6
D) 8
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds has the smallest dipole moment?
A) Li-Cl
B) C-H
C) O-H
D) H-Cl
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has an atom that has an unfilled valence shell of electrons?
A) molecular bromine, Br2
B) fluoride anion, F-
C) ammonia, NH3
D) aluminum trichloride, AlCl3
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an ionic bond?
A) F-F
B) C-H
C) Li-O
D) C-N
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a primary amine?
A) CH3CH2NHCH3
B) CH3CH2NHCH(CH3) 2
C) CH3CH2N(CH3) 2
D) (CH3) 3CNH2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a primary (1 ) alcohol? 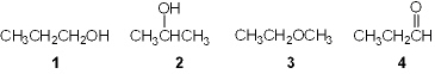
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the tertiary (3 ) amines that have the formula C5H11N.
Correct Answer

verified
11eb135c_c79b_883e_8021_ab361ce75112_TB7077_00
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole? 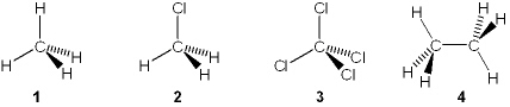
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the C-O bond of dimethyl ether, (CH3) 2O?
A) C 2sp3 + O 2sp2
B) C 2sp2 + O 2p
C) C 2sp2 + O 2sp2
D) C 2sp3 + O 2sp3
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a fluorine atom (fluorine: atomic number 9) ?
A) 1s12s12p7
B) 1s22s22p5
C) 1s22s22p6
D) 1s02s22p7
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the tertiary (3 ) alcohols that have the formula C6H14O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are there in the valence shell of the carbon atom of a methyl cation, CH3+?
A) 4
B) 5
C) 6
D) 7
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding resonance structures?
A) All resonance structures must have the same number of electrons
B) Each atom in all of the resonance structures must have a complete shell of valence electrons
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must be valid Lewis structures
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a fluoride anion (fluorine: atomic number 9) ?
A) 1s22s22p2
B) 1s22s22p5
C) 1s22s22p6
D) 1s22s22p7
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has an atom that has an unfilled valence shell of electrons?
A) molecular hydrogen, H2
B) hydroxide anion, HO-
C) boron trifluoride, BF3
D) water, H2O
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an ester? 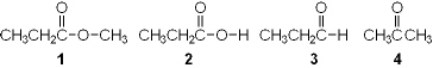
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following compounds is a carboxylic acid?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) CH3CH2CHO
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 95
Related Exams